66085-59-4
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 125 °C |
| Solubility | 1.20e-02 g/L |
| LogP | 3.05 |
| Collision Cross Section | 197.8 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 2840.44 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 418.4 g/mol |
|---|---|
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 418.17400117 g/mol |
| Monoisotopic Mass | 418.17400117 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 736 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
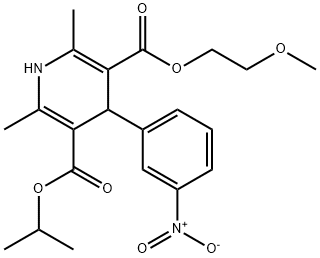
Nimodipine is a dihydropyridine that is 1,4-dihydropyridine which is substituted by methyl groups at positions 2 and 6, a (2-methoxyethoxy)carbonyl group at position 3, a m-nitrophenyl group at position 4, and an isopropoxycarbonyl group at position 5. An L-type calcium channel blocker, it acts particularly on cerebral circulation, and is used both orally and intravenously for the prevention and treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm. It has a role as an antihypertensive agent, a calcium channel blocker, a vasodilator agent and a cardiovascular drug. It is a dihydropyridine, a C-nitro compound, a diester, a member of dicarboxylic acids and O-substituted derivatives, a 2-methoxyethyl ester and an isopropyl ester.
