6398-98-7
Product Name:
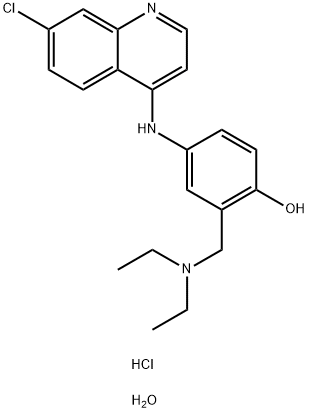
Amodiaquin dihydrochloride dihydrate
Formula:
C20H25Cl2N3O2
Synonyms:
4-([7-Chloro-4-quinolinyl]amino)-2-([diethylamino]methyl)phenol;Amodiaquin dihydrochloride dihydrate
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
COMPUTED DESCRIPTORS
| Molecular Weight | 464.8 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 463.119625 g/mol |
| Monoisotopic Mass | 463.119625 g/mol |
| Topological Polar Surface Area | 50.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 406 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Amodiaquine Hydrochloride is the hydrochloride salt of amodiaquine, an orally active 4-aminoquinoline derivative with antimalarial and anti-inflammatory properties. Similar in structure and activity to chloroquine, amodiaquine is effective against some chloroquine-resistant strains, particularly Plasmodium falciparum, the most deadly malaria parasite. Although the mechanism of plasmodicidal action has not been fully elucidated, like other quinoline derivatives, amodiaquine likely is able to inhibit heme polymerase activity in the body. This results in accumulation of free heme, which is toxic to the parasites.