63074-08-8
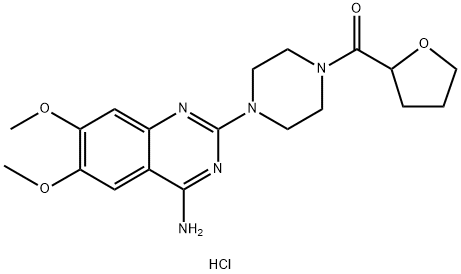
Product Name:
Terazosin hydrochloride
Formula:
C19H26ClN5O4
Synonyms:
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetrahydro-2-furanyl)carbonyl]piperazine hydrochloride;1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetrahydro-2-furanyl)carbonyl]piperazine hydrochloride dihydrate;Terazosin hydrochloride dihydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 195.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 423.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 423.1673320 g/mol |
| Monoisotopic Mass | 423.1673320 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 544 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Terazosin Hydrochloride is the hydrochloride salt form of terazosin, a quinazoline derivative with adrenergic antagonistic property. Terazosin hydrochloride selectively inhibits alpha-1 adrenergic receptors, resulting in vasodilation leading to decreased peripheral vascular resistance and a reduced venous return to the heart as well as decreased urethral resistance, which potentially improving urine flow and symptoms related to benign prostatic hyperplasia. In addition, terazosin decreases low-density lipoproteins (LDL) and triglycerides while increasing the concentration of high-density lipoproteins (HDL).