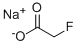CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium fluoroacetate appears as a fine, white, odorless, powdered solid. Toxic by ingestion, inhalation and skin absorption. Used as a rodenticide. |
|---|---|
| Color/Form | White solid |
| Odor | Odorless |
| Boiling Point | Decomposes (NIOSH, 2023) |
| Melting Point | 392 °F Decomposes at 392 °F. (EPA, 1998) |
| Solubility | Miscible (NIOSH, 2023) |
| Density | greater than 1 (temperature not listed) (USCG, 1999) |
| Vapor Pressure | 0 mmHg at 68 °F (EPA, 1998) |
| Stability/Shelf Life | Non-volatile |
| Decomposition | Decomp when heated above melting point. |
| Dissociation Constants | pKa = 2.72 at 25 °C |
| Other Experimental Properties | Hygroscopic |
| Chemical Classes | Pesticides -> Rodenticides |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.02 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 99.99365175 g/mol |
| Monoisotopic Mass | 99.99365175 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 46.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium fluoroacetate appears as a fine, white, odorless, powdered solid. Toxic by ingestion, inhalation and skin absorption. Used as a rodenticide.