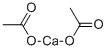CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Liquid; Other Solid; Pellets or Large Crystals |
|---|---|
| Color/Form | COLORLESS CRYSTALS |
| Odor | SLIGHT ODOR OF ACETIC ACID |
| Melting Point | > 160 °C |
| Solubility | 37.4 G IN 100 CC OF WATER @ 0 °C; 29.7 G IN 100 CC OF WATER @ 100 °C; SLIGHTLY SOL IN ALCOHOL |
| Density | 1.50 kg/l |
| Stability/Shelf Life | VERY HYGROSCOPIC ... |
| Decomposition | When heated to decomposition ... emits acrid smoke and fumes. |
| pH | 6,0-9,0 (10 % aqueous solution) |
| Refractive Index | INDEX OF REFRACTION: 1.55 |
| Other Experimental Properties | Decomp above 160 °C to acetone and calcium carbonate |
| Chemical Classes | Metals -> Organic Acids, Metal Salts |
COMPUTED DESCRIPTORS
| Molecular Weight | 158.17 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 157.9891995 g/mol |
| Monoisotopic Mass | 157.9891995 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Calcium acetate is the calcium salt of acetic acid. It is used, commonly as a hydrate, to treat hyperphosphataemia (excess phosphate in the blood) in patients with kidney disease: the calcium ion combines with dietary phosphate to form (insoluble) calcium phosphate, which is excreted in the faeces. It has a role as a chelator. It contains an acetate.