6156-78-1
Product Name:
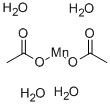
MANGANESE(II) ACETATE TETRAHYDRATE
Formula:
C4H14MnO8
Synonyms:
Manganese(II)acetate tetrahydrate;Manganous acetate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Light red transparent solid; [Merck Index] Light pink hygroscopic crystals; [Acros Organics MSDS] |
|---|---|
| Chemical Classes | Metals -> Organic Acids, Metal Salts |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 245.09 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 245.006910 g/mol |
| Monoisotopic Mass | 245.006910 g/mol |
| Topological Polar Surface Area | 84.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 7 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Manganese triacetate is a chemical compound of manganese. Manganese is a naturally occurring metal with the symbol Mn and the atomic number 25. It does not occur naturally in its pure form, but is found in many types of rocks in combination with other substances such as oxygen, sulfur, or chlorine. Manganese occurs naturally in most foods and small amounts are needed to stay healthy, as manganese ions act as cofactors for a number of enzymes. (L228, L229)