56-04-2
Product Name:
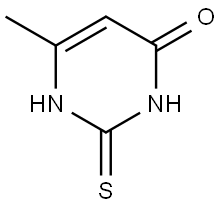
Methylthiouracil
Formula:
C5H6N2OS
Synonyms:
4-Hydroxy-2-mercapto 6-methylpyrimidine;Basethyrin;Methiocil;MZU;Thiothymin
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Methylthiouracil appears as white crystalline powder with an odor of onions and a bitter taste. A saturated aqueous solution is neutral or slightly acidic. (NTP, 1992) |
|---|---|
| Color/Form | CRYSTALS |
| Odor | ODORLESS |
| Taste | BITTER |
| Melting Point | 570 to 577 °F (Decomposes) (NTP, 1992) |
| Solubility | less than 1 mg/mL at 73 °F (NTP, 1992) |
| pH | SATURATED AQ SOLN IS NEUTRAL OR SLIGHTLY ACID TO LITMUS. |
| Collision Cross Section | 123.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | SUBLIMES READILY. |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 142.18 g/mol |
|---|---|
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 142.02008399 g/mol |
| Monoisotopic Mass | 142.02008399 g/mol |
| Topological Polar Surface Area | 73.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 197 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Methylthiouracil appears as white crystalline powder with an odor of onions and a bitter taste. A saturated aqueous solution is neutral or slightly acidic. (NTP, 1992)