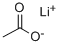CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; Becomes dihydrate at 56.5 deg C; [Merck Index] White powder; Soluble in water; [MSDSonline] |
|---|---|
| Chemical Classes | Metals -> Organic Acids, Metal Salts |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 66.0 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 66.02930777 g/mol |
| Monoisotopic Mass | 66.02930777 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 34.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Lithium acetate is an acetate salt comprising equal numbers of acetate and lithium ions. It is an organic lithium salt and an acetate salt. It contains an acetate.