537-98-4
Product Name:
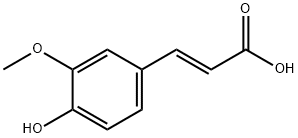
trans-Ferulic acid
Formula:
C10H10O4
Synonyms:
trans-4-Hydroxy-3-methoxycinnamic acid;trans-Ferulic acid;Ferulic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | cis-Form is a yellow liquid; trans-Form is a solid; [Merck Index] trans-Isomer: Tan powder; [Alfa Aesar MSDS] |
|---|---|
| Melting Point | 168 - 171 °C |
| Vapor Pressure | 0.00000269 [mmHg] |
| LogP | log Kow = 1.51 |
| Dissociation Constants | pKa = 4.58 |
| Collision Cross Section | 139.8 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)] |
| Kovats Retention Index | 1897.1 |
| Other Experimental Properties | Yellow oil. UV max (alcohol): 316 nm /cis-Form/ |
| Chemical Classes | Other Classes -> Organic Acids |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 194.18 g/mol |
|---|---|
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 194.05790880 g/mol |
| Monoisotopic Mass | 194.05790880 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 224 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
trans-Ferulic acid was used for esterification of methacrylated dextran. It was also used to study the effect of oral administration of trans-ferulic acid to c57BL mice on ethanol-induced liver injury. Furthermore, it has been used in the synthesis of new biocompatible antioxidant polymers linking trans-ferulic acid.