50512-35-1
Product Name:
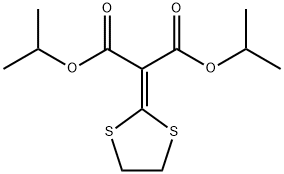
Isoprothiolane
Formula:
C12H18O4S2
Synonyms:
Diisopropyl 2-(1,3-dithiolan-2-ylidene)malonate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 50 - 54.5 °C |
| Solubility | 0.054 mg/mL at 25 °C |
| LogP | 2.88 |
| Kovats Retention Index | 2198 2167 2175 2177 2154.3 2167 2194 2164.2 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 290.4 g/mol |
|---|---|
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 290.06465140 g/mol |
| Monoisotopic Mass | 290.06465140 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 329 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Isoprothiolane is a malonate ester that is diisopropyl malonate in which the two methylene hydrogens at position 2 are replaced by a 1,3-dithiolan-2-ylidene group. An insecticide and fungicide used to control a range of diseases including Pyricularia oryzae, Helminthosporium sigmoideum and Fusarium nivale. It has a role as an insecticide, an environmental contaminant, a phospholipid biosynthesis inhibitor and an antifungal agrochemical. It is a malonate ester, a member of dithiolanes and an isopropyl ester. It is functionally related to a malonic acid. It derives from a hydride of a 1,3-dithiolane.