3902-71-4
Product Name:
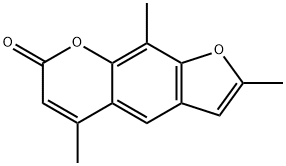
TRIOXSALEN
Formula:
C14H12O3
Synonyms:
2,5,9-Trimethylfuro[3,2-g]benzopyran-7-one;4,5′,8-Trimethylpsoralen;TMP;Trioxsalen;Trisoralen
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 234.5 °C |
| Collision Cross Section | 147.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Kovats Retention Index | 2155 2155 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 228.24 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 228.078644241 g/mol |
| Monoisotopic Mass | 228.078644241 g/mol |
| Topological Polar Surface Area | 39.4 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 374 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Trioxsalen is 7H-Furo[3,2-g]chromen-7-one in which positions 2, 5, and 9 are substituted by methyl groups. Like other psoralens, trioxsalen causes photosensitization of the skin. It is administered orally in conjunction with UV-A for phototherapy treatment of vitiligo. After photoactivation it creates interstrand cross-links in DNA, inhibiting DNA synthesis and cell division, and can lead to cell injury; recovery from the cell injury may be followed by increased melanisation of the epidermis. It has a role as a photosensitizing agent and a dermatologic drug.