358-23-6
Product Name:
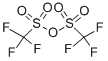
Trifluoromethanesulfonic anhydride
Formula:
C2F6O5S2
Synonyms:
Perfluoromethanesulfonic anhydride solution;Tf2O;Triflic anhydride;Triflic anhydride solution;Trifluoromethanesulfonic anhydride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless hygroscopic liquid; [Merck Index] Clear colorless liquid; [Sigma-Aldrich MSDS] |
|---|---|
| Vapor Pressure | 8.0 [mmHg] |
| Chemical Classes | Other Classes -> Acid Anhydrides, Other |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 282.14 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 2 |
| Exact Mass | 281.90913442 g/mol |
| Monoisotopic Mass | 281.90913442 g/mol |
| Topological Polar Surface Area | 94.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Trifluoromethanesulfonic anhydride finds various applications across different fields of chemistry due to its strong electrophilic nature and its ability to activate functional groups. Trifluoromethanesulfonic anhydride is widely used in organic synthesis as a versatile reagent for various transformations.
RELATED SUPPLIERS
Covalent Incorporation
1Y
product:358-23-6 TRIFLIC ANHYDRIDE (TRIFLUORO MEHTANESULFONIC ANHYDRIDE) 98%