304-21-2
Product Name:
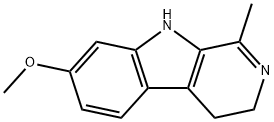
HARMALINE
Formula:
C13H14N2O
Synonyms:
1-Methyl-7-methoxy-3,4-dihydro-β-carboline;3,4-Dihydroharmine;4,9-Dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole;Dihydroharmine;Harmalol methyl ether
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Orthorhombic bipyramidal prisms, tablets from methanol, rhombic octahedra from ethanol |
| Melting Point | 229-231 °C |
| Solubility | 30.6 [ug/mL] (The mean of the results at pH 7.4) |
| Dissociation Constants | pKa = 4.2 |
| Collision Cross Section | 146.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Kovats Retention Index | 2266 |
| Other Experimental Properties | Slender needles, moderately soluble in water, alcohol /Hydrochloride dihydrate/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H371:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 214.26 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 214.110613074 g/mol |
| Monoisotopic Mass | 214.110613074 g/mol |
| Topological Polar Surface Area | 37.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Harmaline is a harmala alkaloid in which the harman skeleton is methoxy-substituted at C-7 and has been reduced across the 3,4 bond. It has a role as a oneirogen. It derives from a hydride of a harman.