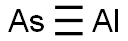SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H331:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 101.90313 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 101.903133 g/mol |
| Monoisotopic Mass | 101.903133 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Aluminum arsenide is a chemical compound of arsenic and aluminum. Arsenic is a chemical element that has the symbol As and atomic number 33. It is a poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and grey forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenopyrite and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as a compound with other elements. Aluminum is the most abundant metal in the earth’s crust and is always found combined with other elements such as oxygen, silicon, and fluorine. (L739, L740, T3)