CHEMICAL AND PHYSICAL PROPERTIES
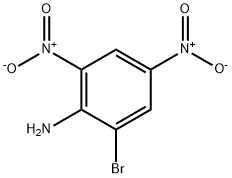
| Physical Description | 2-bromo-4,6-dinitroaniline is a bright yellow powder. (NTP, 1992) |
|---|---|
| Color/Form | YELLOW NEEDLES FROM ACETIC ACID OR ALCOHOL |
| Boiling Point | Sublimes (NTP, 1992) |
| Melting Point | 307 to 309 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 70 °F (NTP, 1992) |
| Other Experimental Properties | SUBLIMES BEFORE REACHING BP |
| Chemical Classes | Nitrogen Compounds -> Nitroanilines |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 262.02 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 260.93852 g/mol |
| Monoisotopic Mass | 260.93852 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 250 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
2-bromo-4,6-dinitroaniline is a bright yellow powder. (NTP, 1992)