170082-17-4
Product Name:
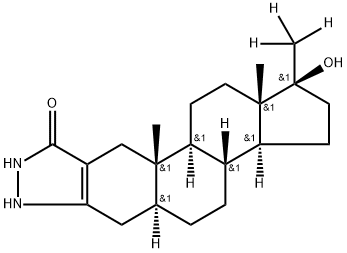
2'H-Androst-2-eno[3,2-c]pyrazol-5'(1'H)-one, 17-hydroxy-17-(methyl-d3)-, (5α,17β)- (9CI)
Formula:
C21H32N2O2
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Methanol appears as a colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Slight alcoholic odor when pure; repulsive, pungent odor when crude |
| Boiling Point | 148.3 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -144 °F (NTP, 1992) |
| Flash Point | 52 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 70 °F (NTP, 1992) |
| Density | 0.792 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 1.11 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 100 mmHg at 70.2 °F ; 237.87 mmHg at 100 °F (NTP, 1992) |
| LogP | log Kow = -0.77 |
| Henry's Law Constant | Henry's Law constant = 4.55X10-6 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | 867 °F (USCG, 1999) |
| Decomposition | Hazardous decomposition products formed under fire conditions: Carbon oxides |
| Viscosity | 0.544 mPa.s at 25 °C |
| Heat of Combustion | 726.1 kJ/mole |
| Heat of Vaporization | 37.34 kJ/mole (at 25 °C) |
| Surface Tension | 22.07 mN/m at 25 °C |
| Ionization Potential | 10.84 eV |
| Odor Threshold | Odor Threshold Low: 4.2 [mmHg] Odor Threshold High: 5960.0 [mmHg] Detection odor threshold from AIHA (mean = 160 ppm) |
| Refractive Index | Index of refraction: 1.3292 at 20 °C/D |
| Dissociation Constants | pKa = 15.3 |
| Kovats Retention Index | 372.7 378.2 400 400 361 368 380 340 384 356 373 330 395 379 373 373 408 381 373 386.1 382 362 381 381 370 354.2 381 353 381 381 348 353 391 384 |
| Other Experimental Properties | Dipole moment: 1.69 /debyes/; specific heat: 0.595-0.605 at 20-25 °C, forms azeotropes with many compounds; burns with nonluminous bluish flame ... |
| Chemical Classes | Solvents -> Alcohols (<C12) |
COMPUTED DESCRIPTORS
| Molecular Weight | 32.042 g/mol |
|---|---|
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 32.026214747 g/mol |
| Monoisotopic Mass | 32.026214747 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Methanol appears as a colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products.