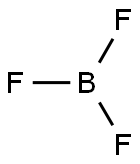| Physical Description |
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material. Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. |
| Color/Form |
Colorless gas |
| Odor |
Pungent, suffocating odor |
| Boiling Point |
-148 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point |
-196.1 °F (EPA, 1998) |
| Solubility |
106 % (in cold H2O) (NIOSH, 2023) |
| Density |
1.6 (EPA, 1998) - Denser than water; will sink |
| Vapor Density |
2.4 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure |
760 mmHg at -149.26 °F Liquid (EPA, 1998) |
| Stability/Shelf Life |
Boron trifluoride ... is stable in dry atmospheres. |
| Decomposition |
Dangerous; when heated to decomposition or upon contact with water or steam, will produce toxic and corrosive fumes of /hydrogen fluoride/. |
| Viscosity |
0.0171 m Pa.s (gas) at 25 °C |
| Corrosivity |
Boron trifluoride will attack some forms of plastics, rubber, and coatings |
| Heat of Vaporization |
19.33 kJ/mol at -101 °C |
| Surface Tension |
17.2 mN/m at -100 °C |
| Ionization Potential |
15.50 eV |
| Polymerization |
The substance will polymerize unsaturated compounds. |
| Odor Threshold |
Odor Threshold Low: 1.5 [ppm] Odor threshold from AIHA |
| Other Experimental Properties |
Forms dense white fumes in moist air; ... reacts with incandescence when heated with alkali metals or alkaline earth metals except magnesium |
| Chemical Classes |
Toxic Gases & Vapors -> Corrosive Gases |