15096-52-3
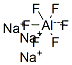
Product Name:
cryolite
Formula:
AlF6Na3
Synonyms:
Kryolith;Sodium hexafluoroaluminate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium aluminum fluoride appears as an odorless white solid or powder. mp: 1291 °C. Density: 2.95 g/cm3. Dust irritates the eyes and skin; inhaled dust irritates the nose, mouth and lungs. Insoluble in water. Synthesized by fusion of sodium fluoride and aluminum fluoride as a electrolyte in the reduction of alumina to aluminum metal. Occurs in nature as the mineral cryolite. Aqueous suspensions of powdered sodium aluminum fluoride are used as insecticides. |
|---|---|
| Color/Form | MONOCLINIC CRYSTALS (NATURAL PRODUCT); AMORPHOUS POWDER (SYNTHETIC PRODUCT) |
| Odor | Odorless. |
| Boiling Point | Decomposes (NIOSH, 2023) |
| Melting Point | 1832 °F (NIOSH, 2023) |
| Solubility | 0.04 % (NIOSH, 2023) |
| Density | 2.9 (NIOSH, 2023) - Denser than water; will sink |
| Vapor Pressure | 0 mmHg (approx) (NIOSH, 2023) |
| Stability/Shelf Life | Low melting solids or colorless, volatile liquids. /Alkylaluminum halides/ |
| Decomposition | When heated to decomposition it emits toxic fumes of /fluorides and disodium oxide/. |
| Heat of Vaporization | 225 kJ/mol at 1012 °C |
| Surface Tension | Liquid in air, 125 mN/m (= dyn/cm) |
| Refractive Index | Index of refraction: 1.338 |
| Other Experimental Properties | Moh's hardness 2.5-3; fuses fairly easily |
| Chemical Classes | Other Classes -> Fluorides, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 209.941265 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 0 |
| Exact Mass | 209.9412652 g/mol |
| Monoisotopic Mass | 209.9412652 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 62.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium aluminum fluoride appears as an odorless white solid or powder. mp: 1291 °C. Density: 2.95 g/cm3. Dust irritates the eyes and skin; inhaled dust irritates the nose, mouth and lungs. Insoluble in water. Synthesized by fusion of sodium fluoride and aluminum fluoride as a electrolyte in the reduction of alumina to aluminum metal. Occurs in nature as the mineral cryolite. Aqueous suspensions of powdered sodium aluminum fluoride are used as insecticides.