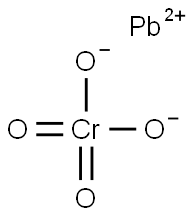
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Lead chromate appears as yellow or orange-yellow powder. One of the most insoluble salts. Basic lead chromates are used as pigments. (NTP, 1992) |
|---|---|
| Color/Form | Yellow or orange-yellow powder |
| Odor | Odorless |
| Boiling Point | Decomposes (NTP, 1992) |
| Melting Point | 1551 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 66 °F (NTP, 1992) |
| Density | 6.12 at 59 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | Approximately 0 mm Hg |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition it emits toxic fumes of /inorganic lead/ |
| pH | Bivalent chromium compounds are basic |
| Refractive Index | Index of refraction: 2.31, 2.37 (Li /lamp/), 2.66 |
| Other Experimental Properties | Lead chromate displays colors that indicate its trimorphism. The stable form is monoclinic and has an orange-yellow color. An unstable tetragonal orange-red form is isomorphous with, and stabilized by, PbMoO4. A second unstable yellow form is rthorhombic, isomorphous with and stabilized by PbSO4. The diversity shown by the lead salt is the key to its versatility as a pigment. |
| Chemical Classes | Metals -> Lead Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 323 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 323.89682 g/mol |
| Monoisotopic Mass | 323.89682 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Lead chromate appears as yellow or orange-yellow powder. One of the most insoluble salts. Basic lead chromates are used as pigments. (NTP, 1992)