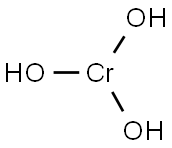
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder |
|---|---|
| Color/Form | Green, gelatinous precipitate |
| Solubility | In an experiment performed under inert gas atmosphere, the solubility /in water/ of freshly precipitated chromium(III) hydroxide was determined from under-saturation and from over-saturation at different pH. As an amphoteric hydroxide, the solubility curve of chromium(III) hydroxide is parabolic, depending on pH. The solubility is as follows: >pH 4: moderately soluble; pH 6.8 - 11.5: insoluble; > pH 11.5 - 14 slightly soluble ... at pH 4, the solubility was about 520 mg/L; at pH 6.8 to 11.8, the solubility was about 0.005 mg/L |
| Decomposition | DECOMP TO CHROMIC OXIDE BY HEAT |
| pH | Trivalent chromium compounds are amphoteric |
| Other Experimental Properties | Blue-green powder; practically insoluble in water, soluble in dilute mineral acids when freshly prepared, giving blue or green solution; becomes insoluble in acids on aging /Chromium trihydroxide trihydrate/ |
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 106.042 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 105.972199 g/mol |
| Monoisotopic Mass | 105.972199 g/mol |
| Topological Polar Surface Area | 3 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 3.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chromium trihydroxide is a hydroxide of chromium. Chromium is a chemical element which has the symbol Cr and atomic number 24. It is found naturally occuring in rocks, animals, plants, and soil, and is usually mined as chromite ore. Chromium is most toxic in its +6 oxidation state (chromium(VI)) due to its greater ability to enter cells and higher redox potential. Trivalent chromium (chromium(III)) however, is biologically necessary for sugar and lipid metabolism in humans. (L17)