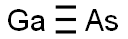CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Gray crystals or powder; [CAMEO] |
|---|---|
| Color/Form | Cubic crystals, dark gray with metallic sheen |
| Odor | Garlic odor when moistened |
| Melting Point | 2260 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 68 °F (NTP, 1992) |
| Density | 5.31 at 77 °F (NTP, 1992) - Denser than water; will sink |
| Decomposition | When heated to decomposition it emits very toxic fumes of /arsenic/. |
| Other Experimental Properties | Hardness 4.5; thermal expansion coefficient: 5.9x10-6; thermal conductivity 0.52 watt units; specific heat 0.086 cal/g/deg c; intrinsic electron concn 10+7; energy gap @ room temp 1.38 electron volts; electron mobility 8800 square cm/volt sec; effective mass for electrons 0.06 m(-0) |
| Chemical Classes | Metals -> Arsenic Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 144.645 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 143.84717 g/mol |
| Monoisotopic Mass | 143.84717 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Gallium arsenide is a chemical compound of gallium and arsenic. It is used to make devices such as microwave frequency integrated circuits, infrared light-emitting diodes, laser diodes and solar cells. It is also a semiconductor. Arsenic is a chemical element that has the symbol As and atomic number 33. It is a poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and grey forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenopyrite and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as a compound with other elements. (T3, L354)