124-41-4
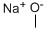
Product Name:
Sodium Methoxide
Formula:
CH3NaO
Synonyms:
Sodium methoxide, Sodium methanolate;Sodium methylate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. It is used to process edible fats and oils, and to make other chemicals. |
|---|---|
| Color/Form | Amorphous, free flowing powder |
| Melting Point | Melting point (decomposes) = 127 °C |
| Solubility | Soluble in ethanol, methanol |
| Density | greater than 1 at 68 °F (USCG, 1999) |
| Stability/Shelf Life | Sensitive to air and moisture |
| Autoignition Temperature | 240 °C |
| Decomposition | Decomposes in air above 126 °C. |
| Other Experimental Properties | Decomposes in air above 127 °C |
| Chemical Classes | Metals -> Metal Alkoxides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H251:Self-heating substances and mixtures H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 54.024 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 54.00815900 g/mol |
| Monoisotopic Mass | 54.00815900 g/mol |
| Topological Polar Surface Area | 23.1 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 4.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. It is used to process edible fats and oils, and to make other chemicals.