12039-13-3
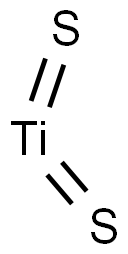
Product Name:
TITANIUM(IV) SULFIDE
Formula:
S2Ti
Synonyms:
Titanium disulphide;Titanium sulfide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Titanium disulfide appears as a yellow or gray powder with an unpleasant odor that is used as a solid lubricant. Contact with the material may cause burns to skin, eyes, and mucous membranes. It may be toxic by ingestion, inhalation or skin absorption. |
|---|---|
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H252:Self-heating substances and mixtures H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P235:Keep cool. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 112.00 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 111.8920830 g/mol |
| Monoisotopic Mass | 111.8920830 g/mol |
| Topological Polar Surface Area | 64.2 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 18.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Titanium disulfide appears as a yellow or gray powder with an unpleasant odor that is used as a solid lubricant. Contact with the material may cause burns to skin, eyes, and mucous membranes. It may be toxic by ingestion, inhalation or skin absorption.