109-99-9
Product Name:
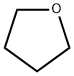
Tetrahydrofuran
Formula:
C4H8O
Synonyms:
THF;Tetrahydrofuran;Oxolane;Butylene oxide;PTHF
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Tetrahydrofuran appears as a clear colorless liquid with an ethereal odor. Less dense than water. Flash point 6 °F. Vapors are heavier than air. |
|---|---|
| Color/Form | Colorless, mobile liquid |
| Odor | Ether-like odor |
| Taste | PUNGENT TASTE |
| Boiling Point | 151 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -163.3 °F (NTP, 1992) |
| Flash Point | 6 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 0.888 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 2.5 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 114 mmHg at 59 °F ; 145 mmHg at 68 °F (NTP, 1992) |
| LogP | log Kow = 0.46 |
| Henry's Law Constant | Henry's Law constant = 7.05X10-5 atm-cu m/mole at 25 °C |
| Stability/Shelf Life | Tetrahydrofuran will auto-oxidize in the presence of light & O2 to form resinous products that color water from pink to brown at conc above 100-250 mg/l. |
| Autoignition Temperature | 610 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits acrid smoke & irritating fumes. |
| Viscosity | 0.53 cP at 20 °C |
| Corrosivity | Tetrahydrofuran will attack some forms of plastics, rubber, and coatings. |
| Heat of Combustion | -14,990 BTU/lb = -8330 cal/g = -348.8X10+5 J/kg |
| Heat of Vaporization | 180 BTU/lb= 98.1 cal/g= 4.1X10+5 J/kg |
| Surface Tension | 26.4 dynes/cm at 25 °C |
| Ionization Potential | 9.45 eV |
| Polymerization | Hazardous polymerization may occur. |
| Odor Threshold | Odor Threshold Low: 0.09 [mmHg] Odor Threshold High: 61.0 [mmHg] Detection odor threshold from AIHA (mean = 31 ppm) |
| Refractive Index | Index of refraction: 1.4050 at 20 °C/D |
| Dissociation Constants | pKa = -2.08 |
| Relative Evaporation Rate | 8 (Butyl acetate = 1) |
| Kovats Retention Index | 617 630 626 609 610 620 622 626 629 619 636 612.5 620 613.7 614.3 615 615 638 608 612 627 615 638 618 |
| Other Experimental Properties | CONVERSION FACTOR: 1 PPM = 2.94 MG/CU M; 1 MG/L = 340 PPM |
| Chemical Classes | Solvents -> Ethers (<C12) |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 72.11 g/mol |
|---|---|
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 72.057514874 g/mol |
| Monoisotopic Mass | 72.057514874 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 22.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tetrahydrofuran appears as a clear colorless liquid with an ethereal odor. Less dense than water. Flash point 6 °F. Vapors are heavier than air. Tetrahydrofuran is a versatile solvent
