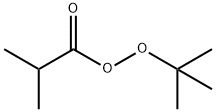
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | This liquid peroxide is particularly sensitive to temperature rises. Above a given "Control Temperature" they decompose violently. It is generally stored or transported in a solvent slurry. Solvent is usually benzene. Responders must regard hazards of the peroxide as well as the benzene solvent. |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 160.21 g/mol |
|---|---|
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 160.109944368 g/mol |
| Monoisotopic Mass | 160.109944368 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
This liquid peroxide is particularly sensitive to temperature rises. Above a given "Control Temperature" they decompose violently. It is generally stored or transported in a solvent slurry. Solvent is usually benzene. Responders must regard hazards of the peroxide as well as the benzene solvent.