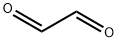CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Glyoxal appears as yellow crystals melting at15 °C. Hence often encountered as a light yellow liquid with a weak sour odor. Vapor has a green color and burns with a violet flame. |
|---|---|
| Color/Form | Yellow prisms or irregular pieces turning white on cooling; opaque at 10 °C; vapors are green |
| Odor | Mild odor |
| Boiling Point | 124 °F at 776 mmHg (NTP, 1992) |
| Melting Point | 59 °F (NTP, 1992) |
| Flash Point | 428 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 72 °F (NTP, 1992) |
| Density | 1.29 at 68 °F (liquid,40% solution) (USCG, 1999) - Denser than water; will sink |
| Vapor Density | greater than 1.0 (NTP, 1992) (Relative to Air) |
| Vapor Pressure | 18 mmHg at 68 °F (NTP, 1992) |
| LogP | -0.85 |
| Henry's Law Constant | Henry's Law constant = 3.33X10-9 atm-cu-m/mol at 25 °C |
| Autoignition Temperature | 545 °F (NTP, 1992) |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| pH | pH of 40% aqueous solution = 2.1 to 2.7 |
| Polymerization | Polymerizes...on contact with water (violent reaction), or when dissolved in solvents containing water. |
| Refractive Index | Index of refraction = 1.3826 at 20 °C |
| Other Experimental Properties | 10.0 lb/gallon at 20 °C; burns with violet flame; aqueous solution contains monomolecular glyoxal and reacts weakly with acid; glyoxal VP resists discoloration; undergoes many addition & condensation reactions with amines, amides, aldehydes & hydroxyl-containing materials. |
| Chemical Classes | Other Classes -> Aldehydes |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 58.04 g/mol |
|---|---|
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 58.005479302 g/mol |
| Monoisotopic Mass | 58.005479302 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 25 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Glyoxal appears as yellow crystals melting at15 °C. Hence often encountered as a light yellow liquid with a weak sour odor. Vapor has a green color and burns with a violet flame.