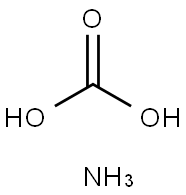
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ammonium carbonate appears as a colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing. |
|---|---|
| Color/Form | Colorless crystalline powder |
| Odor | Strong odor of ammonia |
| Taste | Sharp ammoniacal taste |
| Melting Point | 58 °C (decomposes) |
| Solubility | Soluble in water |
| Density | 1.5 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Decomposes on exposure to air with lose of ammonia and carbon dioxide, becoming white and powdery and converting into ammonium bicarbonate. |
| Decomposition | Decomposes in hot water, yielding ammonia and carbon dioxide. |
| pH | About 8,6 (5 % solution) |
| Odor Threshold | Odor threshold <5 ppm as ammonia gas |
| Other Experimental Properties | Decomposes on exposure to air with loss of NH3 and CO2, becoming white and powdery and converting to ammonium bicarbonate. Volatilizes at about 60 °C. Decomposed by hot water; the carbamate portion dissolves in alcohol |
| Chemical Classes | Nitrogen Compounds -> Ammonium Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 96.09 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 96.05349212 g/mol |
| Monoisotopic Mass | 96.05349212 g/mol |
| Topological Polar Surface Area | 65.2 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium carbonate appears as a colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.