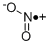CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nitrogen dioxide appears as a reddish brown gas or yellowish-brown liquid when cooled or compressed. Shipped as a liquefied gas under own vapor pressure. Vapors are heavier than air. Toxic by inhalation (vapor) and skin absorption. Noncombustible, but accelerates the burning of combustible materials. Cylinders and ton containers may not be equipped with a safety relief device. |
|---|---|
| Color/Form | Red to brown gas above 21.1 °C; brown liquid below 21.1 °C; colorless solid at approx -11 °C |
| Odor | Irritating odor |
| Boiling Point | 70.07 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 15.3 °F (EPA, 1998) |
| Solubility | Reacts with water (NIOSH, 2023) |
| Density | 1.448 at 68 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 1.58 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 720 mmHg at 68 °F (EPA, 1998) |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitroxides/. |
| Viscosity | 0.0142 at 26.8 °C (gas): 0.42 CP at 20 °C (liquid) |
| Corrosivity | Corrosive to steel when wet, but may be stored in steel cylinders when moisture content is 0.1% or less |
| Heat of Vaporization | 9.110 kcal/mole |
| Ionization Potential | 9.75 eV |
| Odor Threshold | Odor Threshold Low: 0.05 [mmHg] Odor Threshold High: 0.14 [mmHg] Odor threshold from AIHA (odor character = "bleach") |
| Refractive Index | INDEX OF REFRACTION: 1.40 @ 20 °C |
| Other Experimental Properties | Decomposes in water forming nitric acid and nitric oxide, reacts with alkalies to form nitrates and nitrites. |
| Chemical Classes | Toxic Gases & Vapors -> Oxidizers |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H270:Oxidising gases H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P244:Keep reduction valves free from grease and oil. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 46.006 g/mol |
|---|---|
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 45.992903243 g/mol |
| Monoisotopic Mass | 45.992903243 g/mol |
| Topological Polar Surface Area | 30.4 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 7.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nitrogen dioxide appears as a reddish brown gas or yellowish-brown liquid when cooled or compressed. Shipped as a liquefied gas under own vapor pressure. Vapors are heavier than air. Toxic by inhalation (vapor) and skin absorption. Noncombustible, but accelerates the burning of combustible materials. Cylinders and ton containers may not be equipped with a safety relief device.