Fluconazole
Synonym(s):2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol;Fluconazole
- CAS NO.:86386-73-4
- Empirical Formula: C13H12F2N6O
- Molecular Weight: 306.27
- MDL number: MFCD00274549
- EINECS: 627-806-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-16 10:48:58
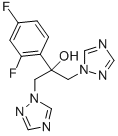
What is Fluconazole?
Background
Fluconazole, commonly known as Diflucan, is an antifungal drug used for the treatment of both systemic and superficial fungal infections in a variety of tissues. It was initially approved by the FDA in 1990. This drug is an azole antifungal, in the same drug family as ketoconazole and itraconazole. Fluconazole has many advantages over the other antifungal drugs including the option of oral administration. The side effect profile of this drug is minimal. It has been demonstrated as an efficacious treatment for vaginal yeast infections in one single dose.
Indications
Fluconazole can be administered in the treatment of the following fungal infections:
1) Vaginal yeast infections caused by Candida
2) Systemic Candida infections
3) Both esophageal and oropharyngeal candidiasis
4) Cryptococcal meningitis
5) UTI (urinary tract infection) by Candida
6) Peritonitis (inflammation of the peritoneum) caused by Candida
A note on fungal infection prophylaxis
Patients receiving bone marrow transplantation who are treated with cytotoxic chemotherapy and/or radiation therapy may be predisposed to candida infections, and may receive fluconazole as prophylactic therapy.
A note on laboratory testing
Obtaining specimens for fungal culture and other important laboratory studies such as serology or pathology is advised before starting fluconazole therapy in order to isolate the organisms to be eliminated through treatment. It is permissible to start therapy before the results are available, however, adjusting the therapy once laboratory results confirm the causative organism may be necessary.
Pharmacokinetics
Fluconazole has been demonstrated to show fungistatic activity against the majority of strains of the following microorganisms, curing fungal infections:
Candida albicans, Candida glabrata (Many strains are intermediately susceptible), Candida parapsilosis, Candida tropicalis, Cryptococcus neoformans
This is achieved through steroidal inhibition in fungal cells, interfering with cell wall synthesis and growth as well as cell adhesion, thereby treating fungal infections and their symptoms.
The fungistatic activity of fluconazole has also been shown in normal and immunocompromised animal models with both systemic and intracranial fungal infections caused by Cryptococcus neoformans and for systemic infections caused by Candida albicans. It is important to note that resistant organisms have been found against various strains of organisms treated with fluconazole. This further substantiates the need to perform susceptibility testing when fluconazole is considered as an antifungal therapy.
A note on steroidal effects of fluconazole
There has been some concern that fluconazole may interfere with and inactivate human steroids/hormones due to the inhibition of hepatic cytochrome enzymes. Fluconazole has demonstrated to be more selective for fungal cytochrome P-450 enzymes than for a variety of mammalian cytochrome P-450 enzymes. Fluconazole 50 mg administered daily for up to 28 days in individuals of reproductive age has been show to have no effect on testosterone plasma concentrations of males and plasma concentrations of steroids in females. A 200-400 mg dose of fluconazole showed no clinically relevant effect on steroid levels or on ACTH-stimulated steroid response in healthy males, in one clinical study mentioned on the European Medicines Agency label. Other studies have shown no significant effects of fluconazole on steroid levels, further confirming these data.
The Uses of Fluconazole
Fluconazole is used to prevent and treat a variety of fungal and yeast infections. It belongs to a class of drugs called azole antifungals. It works by stopping the growth of certain types of fungus.
Mechanism of action
Fluconazole (Diflucan) is an azole antifungal medication that works by stopping the fungus from being able to make a protective covering. This causes the fungus to not grow or survive.
Side Effects
Adverse drug reactions associated with fluconazole therapy include
Common (≥1% of patients): rash, headache, dizziness, nausea, vomiting, abdominal pain, diarrhea, and/or elevated liver enzymes
Infrequent (0.1–1% of patients): anorexia, fatigue, constipation
Rare (<0.1% of patients): oliguria, hypokalaemia, paraesthesia, seizures, alopecia, Stevens–Johnson syndrome, thrombocytopenia, other blood dyscrasias, serious hepatotoxicity including liver failure, anaphylactic/anaphylactoid reactions Very rare: prolonged QT interval, torsades de pointes
Toxicity
Acute oral toxicity (LD50): 1271 mg/kg (rat)
Overdose information
Fluconazole overdoses have been associated with hallucination and paranoia, sometimes in combination. In cases of overdose, employ supportive treatment. Gastric lavage may be necessary. Other modalities such as forced diuresis or hemodialysis may also be used.
A note on liver toxicity
The FDA label warns that this drug carries a risk of hepatotoxicity. Rare but serious cases of serious hepatic toxicity have been reported, especially in patients with serious underlying medical conditions using fluconazole. This group of patients has an increased risk of fatality when using fluconazole. In patients with existing liver dysfunction, use caution during fluconazole therapy. Those who are found to have abnormal liver function tests during therapy should be carefully monitored for the development of increasingly severe injury to the liver. Fluconazole should be stopped if its use is likely to be the underlying cause of liver injury, and medical attention should be sought. Fluconazole induced hepatotoxicity is usually reversible.
Carcinogenesis, mutagenesis, and impairment of fertility
Fluconazole demonstrated no evidence of carcinogenic risk in mice and rats treated orally for 24 months at doses equivalent to approximately 2-7 time the recommended human dose). Male rats given fluconazole at doses equivalent to supratherapeutic human doses showed an increased incidence of hepatocellular adenomas. Cytogenetic studies in vivo and in vitro demonstrated no sign of chromosomal mutation. The significance of these findings for humans is unknown.
Use in pregnancy
There are no sufficient and well-controlled studies of fluconazole use in pregnant women. Available human data do not show an increased risk of congenital anomalies after pregnant women were treated with standard doses (<200 mg/day) of fluconazole, either in a single dose or multiple doses in the first trimester did not appear to impact the fetus negatively. Several case reports describe rare but striking congenital anomalies observed in infants who were exposed to fluconazole at high doses reaching 400-800 mg/day, primarily in the first trimester of pregnancy. Similar findings were observed in animal studies. If this drug is administered during pregnancy, or if the patient becomes pregnant while taking fluconazole, the risk should be discussed thoroughly.
Use in nursing
Fluconazole is secreted in breastmilk at high concentrations. Exercise caution if this drug is used during nursing.
Metabolism
Fluconazole is metabolized minimally in the liver. Fluconazole is an inhibitor of CYP2C9, CYP3A4 and CYP2C19. Two metabolites were detected in the urine of healthy volunteers taking a 50 mg radiolabeled dose of fluconazole; a glucuronidated metabolite on the hydroxyl moiety (6.5%) and a fluconazole N-oxide metabolite (2%). The same study indicated that no signs of metabolic cleavage of fluconazole were observed, suggesting a difference in metabolism when compared to other agents in the same drug class, which are heavily metabolized in the liver.
Properties of Fluconazole
| Melting point: | 138-140°C |
| Boiling point: | 579.8±60.0 °C(Predicted) |
| Density | 1.05 |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: 5 mg/mL |
| form | solid |
| color | White to Off-White |
| Water Solubility | 1g/L(temperature not stated) |
Safety information for Fluconazole
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Fluconazole
| InChIKey | RFHAOTPXVQNOHP-UHFFFAOYSA-N |
Abamectin manufacturer
Jigs Chemical ltd
HEMA PHARMACEUTICALS PVT LTD
NB Healthcare Pvt Ltd
AVD pharmaceuticals Pvt Ltd
SGMR PHARMACEUTICALS PVT LTD
ANWITA
Viruj Pharmaceuticals Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


You may like
-
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 FLUCONAZOLE 99%View Details
FLUCONAZOLE 99%View Details -
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 FLUCONAZOLE 99%View Details
FLUCONAZOLE 99%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details -
 Fluconazole 99%View Details
Fluconazole 99%View Details -
 Fluconazole 98%View Details
Fluconazole 98%View Details